Graduate Research Assistant
University of Missouri-Columbia, Department of Chemistry

All of my life I’ve followed a path of constant inquiry — a path that has lead me through the process of understanding and feeding a sense of curiosity and wonder for what makes the world work. It’s that path that has lead me to the scientific community and will continue to guide my future route of discovery.
In 2009, I completed my bachelor’s in biochemistry with honors in chemistry at Knox College in Galesburg, Illinois. While at Knox, my work focused on developing a fluorescence anisotropy based methodology as a way to better understand in-vitro protein folding dynamics.
As graduate student at the University of Missouri in Columbia, Missouri, I have focused on creating a new boron-dipyrromethene (BODIPY) based dual modality imaging agent for monitoring peptide movement in-vivo. A BODIPY based scaffold holds a great deal of promise as a new dual modality imaging agent because of its excellent fluorescence and biological properties. Furthermore, the existing BF2 motif provides a site for potential radiofluorination.
Outside of chemistry, I am an avid skier and outdoor enthusiast and am a lifelong piano and trombone player.
University of Missouri-Columbia, Department of Chemistry
Brookhaven National Laboratory, Department of Chemistry
Knox College, Department of Chemistry and Biochemistry
University of Missouri-Columbia, Department of Chemistry
Monsanto Corporation
Chemistry (Organic and Radiochemistry)
University of Missouri-Columbia, Department of Chemistry
Biochemistry, Minor in Spanish, Honors in Chemistry
Knox College, Department of Biochemistry
Throughout my research career, I have worked on a wide variety of different projects. Initially, an up bringing in a agricultural community lead me to pursue an internship at Monsanto Corporation. During those two summer internships, 2005 and 2006, I worked with a team of crop physiologists whose goal was to better understand the effects of gene mutations on several different physiological traits.
Later while at Knox College, I transferred my background in biology into developing fluorescence anisotropy based tools to monitor protein folding dynamics. Meanwhile, I was also working under the direction of Tim Glass to develop novel fluorescence sensors for small molecules.
After beginning my Ph.D. in the Glass Group, I began working on a project based on creating novel dual modality (PET/Fluorescence) BODIPY peptide constructs.


During Summer 2005 and 2006, I worked with a group of crop physiologists to monitor changes in overall plant health during the summer growing season. Growing up in a rural community meant that my first exposure to the scientific community was through agricultural. My two summers interning at Monsanto as part of their crop physiology team is clear extension of that initial interest. While at Monsanto, I worked with a team of scientists whose goal was to better understand the effects of gene mutations on several physiological traits in corn, cotton, and soybeans. As a crop physiology intern, my task was to monitor and help evaluate several different crop traits in the fields. To carry out this work, I used a modular photosynthesis tool called a LiCor(R). This tool allowed us to collect a variety of types of data relating to overall plant health. After the data was collected, it was analysed looking for overall trends in plant health to help other members of the team decide which construct to pursue in the future.
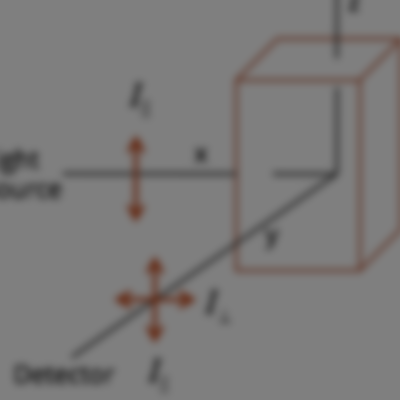
As part of my undergraduate research at Knox College (2009, BA in Biochemistry (with Honors in Chemistry)), I worked with Dr. Larry Welch and Dr. Andrew Mehl developing a fluorescence anisotropy based method to monitor protein folding dynamics via the Perrin equation in vitro.
Fluorescence anisotropy is a powerful fluorescence based spectroscopic technique which allow users to monitor changes in plane polarized light as it relates to a fluorophore or a fluorophore contain biomolecule. The concept of polarization stems a basic first principle of fluorescence which is that during the excitation of ground state electrons only those molecules whose dipole moments are at that moment aligned with incident light are excited.
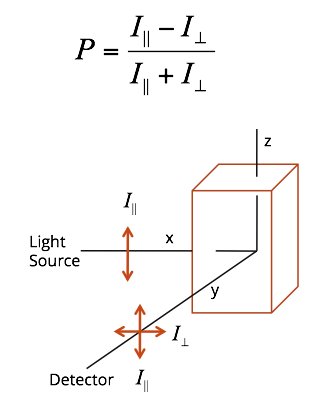
Under normal conditions we don’t restrict the orientation (or polarization) of the light coming into the sample. However, fluorescence anisotropy allows the user to filter away all but a single orientation of flight, and therefore only those molecules whose orientation is aligned with incoming light are excited. During the excited state (i.e the fluorescence lifetime) some or all of that rotation may be retained by the fluorophore. These difference are what we measure during fluorescence anisotropy experiments.
In our case we hoped to use a modified version of the Perrin equation as means to determine the molecular volume of a given protein in solution, and then eventually monitor protein folding dynamics. Our idea was to use the tryptophan residue as a sort of ‘reporter fluorophore’ for detecting changes in a proteins structural motifs. Initially we undertook several proof concept molecules (fluorescein, L-Tryptophan, glucagon, and subtilisin carlsberg) as way to see if we could accurately calculate molecular volume of a known set of standards. Based on our initial set of experimental parameters it was apparent that our methodology worked well for monitoring changes only in cases where the fluorophore or the fluorescent residue was solvent facing. Current work in the Welch is still focused on developing methodologies for better use fluorescence anisotropy to determine protein dynamics.
With that information in mind we decided to start to investigate GrpE, a bacterial heat shock protein. We thought that GrpE would be a good model to start with because it lacks any native fluorescent amino acids which would make spectral deconvolution much more difficult. Furthermore, GrpE has several distinct independent secondary structures which could also be studied in the future, so of which had already been already isolated by previous members of the Mehl lab.
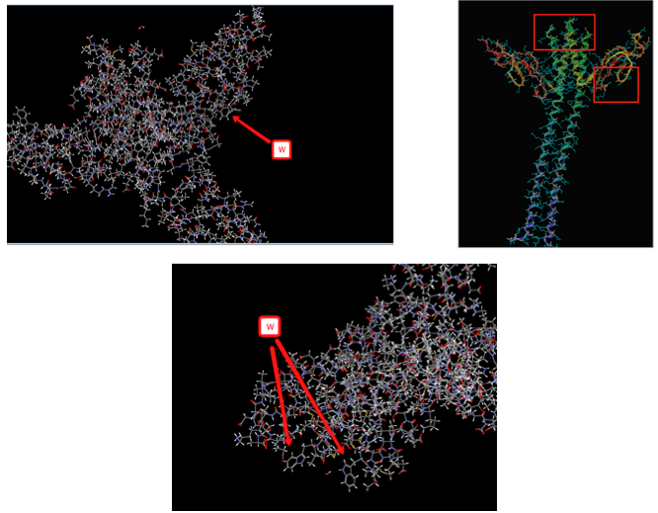
With the help of Acerrlys Discovery Pro (V. 2.1) we identified two possible sites of point mutation (V109W and T145W) which seemed unlikely to cause majors changes in either the protein’s secondary or tertiary structure. Furthermore, both sites seems to have a relatively high amount of solvent exposure making them good choices for our current methodology. However, expression (specifically amplification) and purification of these point mutants proved problematic in our hands, Mehl group members are currently working to develop better expression and purification methods for these GrpE point mutants.
Below is a current list of publications, updated March 2014.
I would be happy to talk to you if you need my assistance in your research or whether you need bussiness administration support for your company. Though I have limited time for students but I Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.